June 2024:
RESEARCH HIGHLIGHTSJingshun Luo
Institute of Molecular Physiology, Shenzhen Bay Laboratory
Abstract:
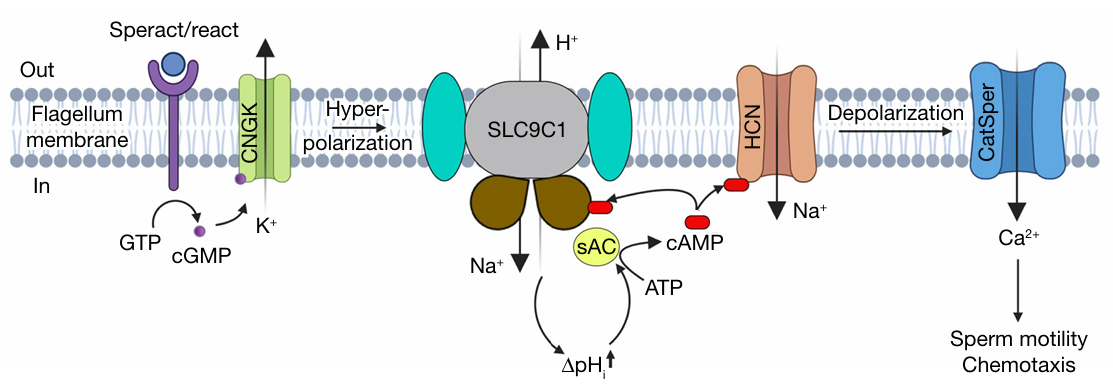
Voltage-sensing domains is believed to control the activation of voltage-gated ion channels. One exception is the sperm-specific Na+/H+ exchanger SLC9C1, which is the only known transporter to be regulated by voltage-sensing domains. Hyperpolarization of sperm flagella activates SLC9C1, which causes pH alkalinization and initiates sperm motility and fertilization. SLC9C1’s activation is additionally regulated by cAMP. Thus, SLC9C1 plays a pivotal role as the integrator of membrane voltage and intracellular cAMP. Despite its importance, the molecular mechanism of SLC9C1 is unclear. Here the authors report the cryo-EM structures of sea urchin SLC9C1. They show that the voltage-sensing domains (VSDs) sandwiches the SLC9C1 homodimer. The VSDs connects the cytoplasmic cyclic-nucleotide-binding domains via a 90-Å S4 segment. In inactive state of SLC9C1, the upward S4 segment locks the negatively charged cavity for Na+ transportation. Upon hyperpolarization, the downward S4 segment releases this constriction and enables Na+/H+ exchange.
Graphical Abstract or Main Figure
Text:
Directed sperm motility is essential for fertilization. From corals to human, metazoan sperm mobility is directed by a highly conserved signaling cascade. The sperm motility is triggered by the pH-dependent activation of the cAMP-producing enzyme soluble adenylyl cyclase (sAC)1, then cAMP activates SLC9C1. SLC9C1 is essential for male fertility and its expression correlates with sperm count and motility 2. SLC9C1 belongs to the SLC9 Na+/H+ exchangers (NHEs) superfamily, the members of which are essential for the regulation of intracellular pH and sodium homeostasis3. However, the structure of SLC9C1 remains unclear. To fill the blank in this area, Kalienkova et al. resolve the structure of SLC9C1 by Cryo-EM, and characterize its function with solid-supported membrane (SSM)-based electrophysiology. They selected the sea urchin Strongylocentrotus purpuratus as the model organism, given its long history in the study fertilization. The SLC9C1 from the sea urchin shares around 30% sequence identity with human SLC9C14.
The authors succeeded in purifying SLC9C1 expressed in HEK293 cells and reconstituted the protein into liposomes made from yeast polar lipids. To characterize the function of SLC9C1, they mounted the proteo-liposomes onto the SSM, and conducted electrophysiology recording 5. They found that the peak currents of the SLC9C1-reconstituted liposomes were significantly higher than the that obtained for either empty liposomes or liposomes with mutant SLC9C1. Importantly, they found that the highest activity for SLC9C1 was observed at pH 6.5, which is close to the sea urchin sperm flagella cytoplasmic pH of 6.7.
The purified SLC9C1 was further subjected for cryo-EM structure analysis. The results showed that SLC9C1 has a transport module (Na+ /H+ exchanger, NHE), a voltage-sensing domain (VSD) and a cyclic-nucleotide binding domain (CNBD). This combination of domains is not found in any other membrane protein described to date.
The NHE transporter module of the SLC9C1 monomer consists of 13 TM segments, and resembles the structures of mammalian NHE1, NHE3 and NHE9 6,7. The structure of SLC9C1 shares the highest structural similarity to human SLC9A1 (NHE1) and horse SLC9A9 (NHE9), both of which were captured in an inward-facing conformation. Overall, the structures of the transporter module and ion-binding sites of SLC9C1 generally fit those of a typical mammalian NHE, with most of differences reside at the oligomerization interface.
The VSDs in SLC9C1 have the classical VSD architecture8. The VSDs in SLC9C1 were surprisingly most similar to VSDIV from the human voltage-gated Na+channel structure of NaV1.7 in the active state. Previous literatures suggest that the voltage sensor in the VSD moves upon changes in the membrane electric field, thereby allows passive ion flux by either directly dilating the pore or releasing an obstruction underneath the pore 9,10. Accordingly, the authors deduced that upon hyperpolarization, specific domain in the VSD region of SLC9C1 will undergo displacement thereby allow ion exchange through the NHE region.
The CNBD in SLC9C1 resembles the CNBD seen in HCN channels [11]. cAMP primes SLC9C1 for activation by detaching CNBD interactions with the cytoplasmic end of S4, thus enable SLC9C1’s activating threashold be closer to the resting membrane potential of sperm (−50 mV).
There are a few examples of non-channel VSD domains coupled to soluble enzymes, and they are thought to have evolved independently from ion channels. This work resolves the structure and function of the only known VSD-regulated transporter SLC9C1, which is essential for sperm motility and fertilization. It also provides generic mechanistic insights that are applied to broad ion transporters and channels.
Link to the research article:
https://www.nature.com/articles/s41586-023-06518-2
1. Speer KF, Allen-Waller L, Novikov DR, Barott KL: Molecular mechanisms of sperm motility are conserved in an early-branching metazoan. P Natl Acad Sci USA 2021, 118(48).
2. Wang D, King SM, Quill TA, Doolittle LK, Garbers DL: A new sperm-specific Na+/H+ Exchanger required for sperm motility and fertility. Nat Cell Biol 2003, 5(12):1117-1122.
3. Romero F, Nishigaki T: Comparative genomic analysis suggests that the sperm-specific sodium/proton exchanger and soluble adenylyl cyclase are key regulators of CatSper among the Metazoa. Zool Lett 2019, 5.
4. Windler F, Bönigk W, Körschen HG, Grahn E, Strünker T, Seifert R, Kaupp UB: The solute carrier SLC9C1 is a Na/H-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding (vol 9, 2809, 2018). Nat Commun 2020, 11(1).
5. Bazzone A, Barthmes M, Fendler K: SSM-Based Electrophysiology for Transporter Research. Method Enzymol 2017, 594:31-83.
6. Winklemann I, Matsuoka R, Meier PF, Shutin D, Zhang C, Orellana L, Sexton R, Landreh M, Robinson CV, Beckstein O et al: Structure and elevator mechanism of the mammalian sodium/proton exchanger NHE9. Embo J 2020, 39(24).
7. Dong YL, Li H, Ilie A, Gao YW, Boucher A, Zhang XJC, Orlowski J, Zhao Y: Structural basis of autoinhibition of the human NHE3-CHP1 complex. Sci Adv 2022, 8(21).
8. Tan XF, Bae C, Stix R, Fernandez-Marino AI, Huffer K, Chang TH, Jiang JS, Faraldo-Gómez JD, Swartz KJ: Structure of the Shaker Kv channel and mechanism of slow C-type inactivation. Sci Adv 2022, 8(11).
9. Catterall WA: Ion Channel Voltage Sensors: Structure, Function, and Pathophysiology. Neuron 2010, 67(6):915-928.
10. Catterall WA, Wisedchaisri G, Zheng N: The chemical basis for electrical signaling. Nat Chem Biol 2017, 13(5):455-463.
11. Lee CH, MacKinnon R: Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 2017, 168(1-2):111-+.
References
1. Speer KF, Allen-Waller L, Novikov DR, Barott KL: Molecular mechanisms of sperm motility are conserved in an early-branching metazoan. P Natl Acad Sci USA 2021, 118(48).
2. Wang D, King SM, Quill TA, Doolittle LK, Garbers DL: A new sperm-specific Na+/H+ Exchanger required for sperm motility and fertility. Nat Cell Biol 2003, 5(12):1117-1122.
3. Romero F, Nishigaki T: Comparative genomic analysis suggests that the sperm-specific sodium/proton exchanger and soluble adenylyl cyclase are key regulators of CatSper among the Metazoa. Zool Lett 2019, 5.
4. Windler F, Bönigk W, Körschen HG, Grahn E, Strünker T, Seifert R, Kaupp UB: The solute carrier SLC9C1 is a Na/H-exchanger gated by an S4-type voltage-sensor and cyclic-nucleotide binding (vol 9, 2809, 2018). Nat Commun 2020, 11(1).
5. Bazzone A, Barthmes M, Fendler K: SSM-Based Electrophysiology for Transporter Research. Method Enzymol 2017, 594:31-83.
6. Winklemann I, Matsuoka R, Meier PF, Shutin D, Zhang C, Orellana L, Sexton R, Landreh M, Robinson CV, Beckstein O et al: Structure and elevator mechanism of the mammalian sodium/proton exchanger NHE9. Embo J 2020, 39(24).
7. Dong YL, Li H, Ilie A, Gao YW, Boucher A, Zhang XJC, Orlowski J, Zhao Y: Structural basis of autoinhibition of the human NHE3-CHP1 complex. Sci Adv 2022, 8(21).
8. Tan XF, Bae C, Stix R, Fernandez-Marino AI, Huffer K, Chang TH, Jiang JS, Faraldo-Gómez JD, Swartz KJ: Structure of the Shaker Kv channel and mechanism of slow C-type inactivation. Sci Adv 2022, 8(11).
9. Catterall WA: Ion Channel Voltage Sensors: Structure, Function, and Pathophysiology. Neuron 2010, 67(6):915-928.
10. Catterall WA, Wisedchaisri G, Zheng N: The chemical basis for electrical signaling. Nat Chem Biol 2017, 13(5):455-463.
11. Lee CH, MacKinnon R: Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 2017, 168(1-2):111-+.