June 2024:
RESEARCH HIGHLIGHTSZhen Xu
Institute of Molecular Physiology, Shenzhen Bay Laboratory
Abstract:
Penile erection, a crucial process for maintaining sexual function, requires the coordinated involvement of vascular and neural components. Parasympathetic nerves release nitric oxide and acetylcholine, promoting relaxation of vascular smooth muscle cells and leading to erection. Conversely, sympathetic nerves release norepinephrine, maintaining contraction of vascular smooth muscle cells and inhibiting erection. Previous research has focused primarily on the roles of penile smooth muscle cells and endothelial cells, while the abundant fibroblasts within the corpus cavernosum (CC) have been largely overlooked. This study elucidates the role of CC fibroblasts in regulating penile blood flow. Single-cell gene expression profiling and histological analysis identified the specific marker Slc1a3 expressed by CC fibroblasts. Using optogenetic-induced depolarization of fibroblasts, we discovered that fibroblasts also play a significant role in regulating penile blood flow. Further investigation revealed that CC fibroblasts modulate penile blood flow by regulating the availability of norepinephrine, and the efficacy of this process depends on the number of fibroblasts. The number of fibroblasts can be remodeled with increased frequency of penile erections. During erection, the Notch signaling pathway in CC fibroblasts is inhibited, leading to an increase in fibroblast numbers, which can result in priapism. Aging, a major risk factor for erectile dysfunction, reduces the number of penile fibroblasts and limits penile blood flow. Reduced fibroblast numbers in young animals mimic the penile blood flow phenotype observed in aged animals.
Text:
Penile erection is achieved through the vasodilation of the corpus cavernosum (CC), a trabecular vascular structure extending from the base of the penis to the glans penis1. The balance between vasodilators nitric oxide (NO), acetylcholine and the vasoconstrictor norepinephrine is crucial for the initiation and maintenance of penile erection. Recent studies have identified the presence of fibroblasts in the human CC2,3, but their role in blood flow regulation has not yet been investigated.
The authors first identified that SLC1A3 is specifically expressed in fibroblasts of the CC through single-cell RNA sequencing. They categorized these fibroblasts into three subpopulations: Dnah5 (cluster 1), Cxcl12 (cluster 2), and Gja1 (cluster 3). Cells from clusters 1 and 2 are located near the vasculature within the CC, while cluster 3 cells are primarily found in the avascular outer tunica albuginea of the penis. SLC1A3 has previously been used to label perivascular cells in the central nervous system 4, which aligns with the authors' research background.
To interrogate the function the SLC1A3-positive fibroblasts, the authors conducted optogenetic experiments with the mice expressing the light-sensitive ion channel protein Channelrhodopsin-2 (ChR2) within the Slc1a3-CreERT-labeled cells. Concomitantly, Penile blood flow was non-invasively assessed using high-resolution laser speckle contrast imaging. The blood flow increased significantly upon light activation, and decreased with the retraction of light. In contrast to the slow and sustained blood flow increase induced by the depolarization of penile fibroblasts, the increase in blood flow induced by nitric oxide (NO) or acetylcholine is strong and immediate. These two distinct hemodynamic responses reveal a novel mechanism of penile erection.
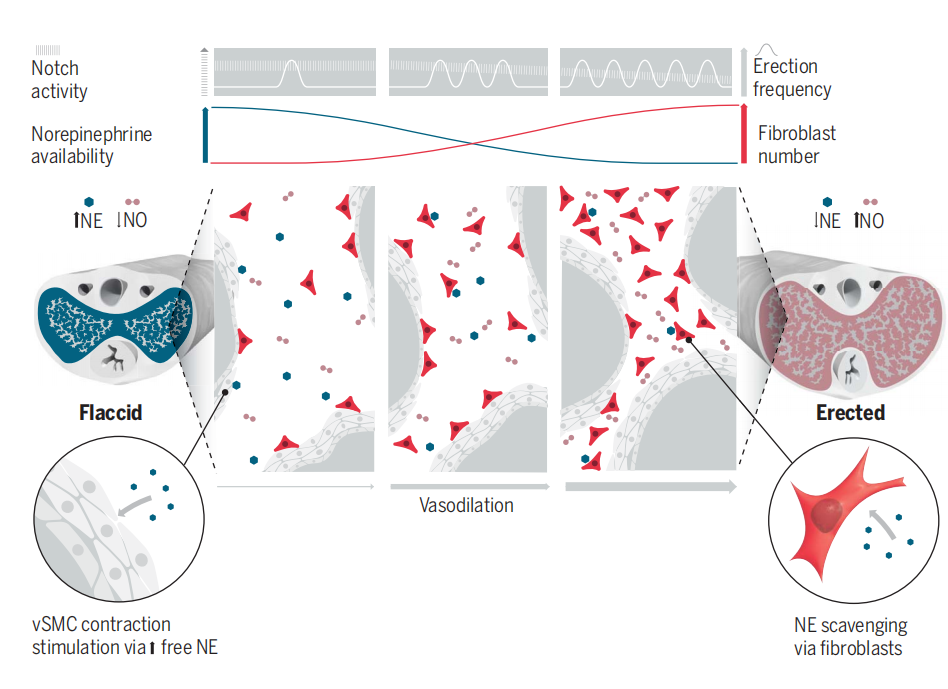
The authors then investigated the mechanisms by which depolarization of CC fibroblasts can lead to penile erection. In the flaccid state, penile blood flow is maintained at a basal level by the presence of the vasoconstrictor norepinephrine. Continuous depolarization of fibroblasts leads to a steady reduction in norepinephrine levels, which may account for the observed increase in penile blood flow. The authors discovered that CC fibroblasts express SLC6A2, a protein that transports norepinephrine into cells, preventing its binding to receptors5. Additionally, these fibroblasts express monoamine oxidase A (MAO-A), an enzyme crucial for norepinephrine degradation6. Through in vitro experiments, the authors demonstrated that penile corpus cavernosum fibroblasts can indeed uptake the norepinephrine analog FFN270. This suggests that penile corpus cavernosum fibroblasts facilitate vasodilation through SLC6A2-mediated norepinephrine uptake.
Notch signaling coordinates cell turnover and blood flow in bone through dynamic changes in cell-to-cell contact and may serve as an indicator for the transition between flaccid and erect states7,8. The authors discovered the expression of Notch1 and Notch2 receptors, the Notch ligand Jagged 1, and the downstream effector RBP-Jk in fibroblasts throughout the CC. Using CBF-H2B-Venus mice, they assessed Notch signaling activity at single-cell resolution and found that Notch signaling is transiently activated in CC fibroblasts during penile erection.
The authors investigated the relationship between the Notch signaling pathway and penile erection. Continuous stimulation of the cavernous nerve induced frequent erections, and further leading to downregulation of Notch signaling in CC fibroblasts. Universal inhibition of Notch signaling increased the number of CC fibroblasts. To specifically reduce Notch signaling in CC fibroblasts, the authors conditionally deleted RBP-Jk, a DNA-binding protein essential for canonical Notch signaling 9. Following RBP-Jk deletion, the penile body and glans penis exhibited higher basal blood flow with increased numbers of CC fibroblasts. In animals with increased numbers of CC fibroblasts, exposure to norepinephrne resulted in a less pronounced reduction in blood flow, suggesting that the number of CC fibroblasts will regulate penile blood flow.
Finally, the authors investigated the changes in penile tissue following the conditional ablation of cavernous fibroblasts in heterozygous mice. The reduction of fibroblasts in the CC of young animals led to decreased basal penile blood flow, akin to observations in aged animals.
Comment:
The authors have elucidated a novel mechanism of penile erection. However, the erection mediated by cavernous fibroblasts is slow and may not facilitate successful intercourse. Thus, whether this new mechanism could be applied in clinical practice warrants further investigation.
Link to the research article:
https://www.science.org/doi/10.1126/science.ade8064
1. Cunha GR, Sinclair A, Ricke WA, et al (2019) Reproductive tract biology: Of mice and men. Differentiation 110:49–63.
2. Zhao L, Han S, Su H, et al (2022) Single-cell transcriptome atlas of the human corpus cavernosum. Nat Commun 13:4302.
3. Fang D, Tan X-H, Song W-P, et al (2022) Single-Cell RNA Sequencing of Human Corpus Cavernosum Reveals Cellular Heterogeneity Landscapes in Erectile Dysfunction. Front Endocrinol (Lausanne) 13:874915.
4. Dias DO, Kalkitsas J, Kelahmetoglu Y, et al (2021) Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun 12:5501.
5. Cheng MH, Bahar I (2019) Monoamine transporters: structure, intrinsic dynamics and allosteric regulation. Nat Struct Mol Biol 26:545–556.
6. Jones DN, Raghanti MA (2021) The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J Chem Neuroanat 114:101957.
7. Ramasamy SK, Kusumbe AP, Wang L, Adams RH (2014) Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507:376–380.
8. Ramasamy SK, Kusumbe AP, Schiller M, et al (2016) Blood flow controls bone vascular function and osteogenesis. Nat Commun 7:13601.
9. Bray SJ (2016) Notch signalling in context. Nat Rev Mol Cell Biol 17:722–735.
References
1. Cunha GR, Sinclair A, Ricke WA, et al (2019) Reproductive tract biology: Of mice and men. Differentiation 110:49–63.
2. Zhao L, Han S, Su H, et al (2022) Single-cell transcriptome atlas of the human corpus cavernosum. Nat Commun 13:4302.
3. Fang D, Tan X-H, Song W-P, et al (2022) Single-Cell RNA Sequencing of Human Corpus Cavernosum Reveals Cellular Heterogeneity Landscapes in Erectile Dysfunction. Front Endocrinol (Lausanne) 13:874915.
4. Dias DO, Kalkitsas J, Kelahmetoglu Y, et al (2021) Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat Commun 12:5501.
5. Cheng MH, Bahar I (2019) Monoamine transporters: structure, intrinsic dynamics and allosteric regulation. Nat Struct Mol Biol 26:545–556.
6. Jones DN, Raghanti MA (2021) The role of monoamine oxidase enzymes in the pathophysiology of neurological disorders. J Chem Neuroanat 114:101957.
7. Ramasamy SK, Kusumbe AP, Wang L, Adams RH (2014) Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507:376–380.
8. Ramasamy SK, Kusumbe AP, Schiller M, et al (2016) Blood flow controls bone vascular function and osteogenesis. Nat Commun 7:13601.
9. Bray SJ (2016) Notch signalling in context. Nat Rev Mol Cell Biol 17:722–735.