March 2024:
RESEARCH HIGHLIGHTSWuqiang ZHAN
TMEM63s protein was identified as mechanically activated (MA) ion channels only recently, and play critical roles in normal central nervous system development and neurodegenerative diseases. Here, Zheng et al. solved the cryo-EM structure of HsTMEM63B and HsTMEM63B in uniquely monomeric configuration, whereas OSCA1.2 forms a dimer. The structural and biochemical analysis reveal that the monomeric configuration of TMEM63s is governed by the IL2 domain. The monomeric configurations of TMEM63s induce less favorable conformations of mechanosensing domain IL2 and gating helix TM6 to activate the channel, resulting in high-threshold mechanosensitivties.
OSCA/TMEM63 proteins, the largest family of mechanically activated (MA) ion channels identified, are conserved across eukaryotes1. OSCA channels exist in plants with 15 paralogs identified in Arabidopsis thaliana. OSCAs are bona fide pore-forming mechanosensitive ion channels with preferred selectivity for cations. TMEM63 proteins, including A, B, and C members, are animal orthologs of the plant OSCAs. The recently clinical reports and genetic studies have demonstrated that variants of TMEM63s associated in disorder of central nervous system development and neurodegenerative diseases2,3,4. When expressed in heterologous cells, TMEM63A and TMEM63B revealed small stretch-activated currents, whereas no current was detected for TMEM63C1. The fundamental question is whether TMEM63s are bona fide pore-forming MA ion channels?
Different from the dimeric assembly of OSCAs, both structure of TMEM63A and TMEM63B were resolved in monomer without any symmetry5,6. The overall arrangement of the TMEM63s monomer structure resemble OSCA1.2 protomer subunit. The TMD of TMEM63s contains 11 TM helices and ICD of TMEM63s is mainly composed of the IL2 domain, that contains four-stranded anti-parallel β-sheet and five helices.
However, it remains to be confirmed whether TMEM63s are monomer under physiological conditions. Zheng et al. confirmed that the endogenous TMEM63s in mouse brain exists as a monomer by using denaturing and blue native PAGE. In addition, they confirmed that the oligomeric state of TMEM63s is monomer in situ in the cell surface membrane by conducting single-molecule analysis using total internal reflection fluorescence (TIRF) microscopy. However, using Cryo-EM analysis of TMEM63B, Qin et al. observed dimerized TMEM63B which confirmed that TMEM63B is a mix of monomer and dimer in cells7.
Consistently with the previously described for TMEM63A and TMEM63B evoking small currents1, Zheng et al. illustrate that TMEM63A (Imax = 33.5 ± 7.5 pA; n = 6) and TMEM63B (Imax = 17.4 ± 2.3 pA; n = 5) revealed small stretch-activated currents, whereas OSCA1.2 were 10-fold larger (Imax = 310.2 ± 85.1 pA; n = 8), when overexpressed in heterologous cells. In addition, when purified monomers were reconstituted into liposomes, TMEM63A (Imax = 18.7 ± 3.1 pA; n = 6) and TMEM63B (Imax = 16.3 ± 1.8 pA; n = 6) also induced small currents by stretch stimulation. Thus, these results demonstrated that monomeric TMEM63A and TMEM63B are bona fide pore-forming MA ion channels.
The next question is how monomeric TMEM63A and TMEM63B activated by mechanical force? HsTMEM63A or MmTMEM63B expression in HEK293T cells exhibited higher threshold mechanosensitivities than OSCA1.2. Does the oligomeric state affect mechanosensitivity of OSCA/TMEM63s? Structural analysis revealed that the dimerization of OSCA1.2 is solely mediated by the IL2 domain8. Monomeric variants of OSCA1.2, OSCA1.25Mu expressed in HEK293T evoked robust stretch-activated currents (Imax = 171.6 ± 25.5 pA; n =12) with rapid activation and fast inactivation, akin to WT OSCA1.2. These demonstrate that monomeric OSCA1.2 preserves mechanosensitive channel function. Significantly, compared with OSCA1.2, OSCA1.25Mu showed a substantially higher P50 (- 109.8 ± 4.1 mmHg; n = 12), suggesting that switching from a dimer to monomer leads to high-threshold mechanosensitivity.
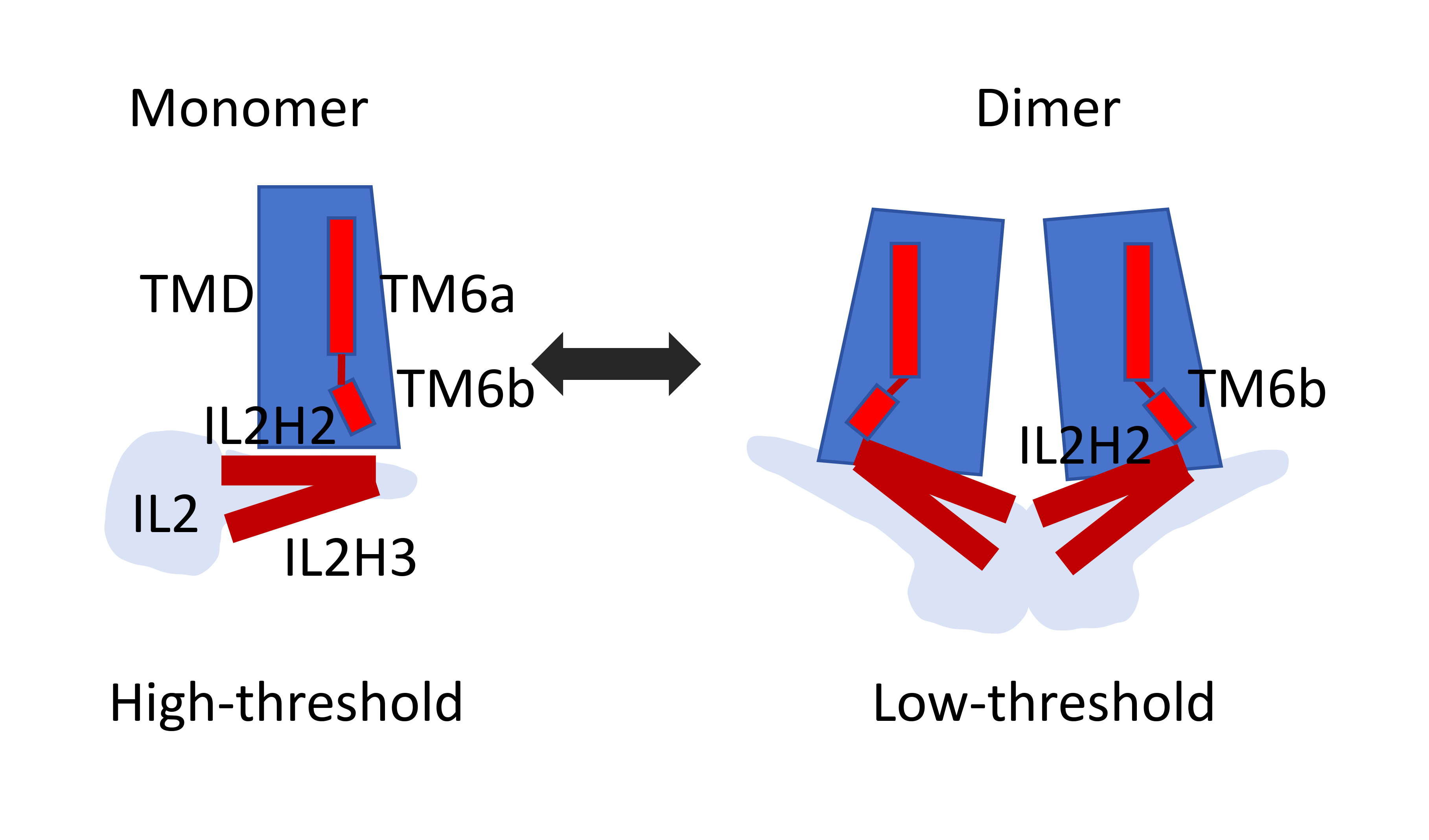
Fig.1 Schematic model showing distinct conformations of IL2 and TM6 in monomeric and dimeric OSCA/TMEM63 proteins.
Comparing structures of monomeric TMEM63s and dimeric OSCA1.2, IL2 domain exhibited more intimate contact with the membrane in OSCA1.2. Then, Zheng et al. speculated that dimerization of OSCA1.2 induces a rigid-body movement of the IL2 domain toward the membrane and stabilizes the IL2 domain in a conformation that allows membrane association of the hypothetic mechano-sensing elements, including amphipathic helices IL2H2/IL2H3 and the hook domain. However, the deletion of the hook domain in OSCA1.2, TMEM63A and TMEM63B had little effect on the mechanosensitivity of these channels. Thus, given the prevalence of the amphipathic helix in other mechanosensitive ion channels, Zheng et al. proposed that the amphipathic helices IL2H2/IL2H3 may serve as the mechano-sensor. Compared to OSCA1.2, the IL2 domain in TMEM63s is largely mobile and mainly adopts a conformation in which IL2H2/IL2H3 is farther from the internal membrane surface, which may confer high-threshold mechanosensitivity.
The study by zheng et al. is a great example of activation mechanisms of monomeric TMEM63s protein. The structural and biochemical analysis reveal that mechanosensitivity in OSCA/TMEM63 channels is affected by oligomerization and suggest interesting gating mechanisms that may be shared by OSCA/TMEM63, TMEM16, and TMC channels (Fig.1).
1. MURTHY S E, DUBIN A E, WHITWAM T, et al. OSCA/TMEM63 are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels [J]. Elife, 2018, 7.
2. FUKUMURA S, HIRAIDE T, YAMAMOTO A, et al. A novel de novo TMEM63A variant in a patient with severe hypomyelination and global developmental delay [J]. Brain and Development, 2022, 44(2): 178-183.
3. VETRO A, BALESTRINI S, PELOROSSO C, et al. Stretch-activated ion channel TMEM63B associates with developmental and epileptic encephalopathies and progressive neurodegeneration [J]. medRxiv, 2022.
4. TABARA L C, AL-SALMI F, MAROOFIAN R, et al. TMEM63C mutations cause mitochondrial morphology defects and underlie hereditary spastic paraplegia [J]. Brain, 2022, 145(9): 3095-3107.
5. ZHANG M, SHAN Y, COX C D, et al. A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity [J]. Nat Commun, 2023, 14(1): 3943.
6. ZHENG W, RAWSON S, SHEN Z, et al. TMEM63 proteins function as monomeric high-threshold mechanosensitive ion channels [J]. Neuron, 2023, 111(20): 3195-3210 e3197.
7. Cameron, C.M., R.M. Wightman, and R.M. Carelli, Dynamics of rapid dopamine release in the nucleus accumbens during goal-directed behaviors for cocaine versus natural rewards. Neuropharmacology, 2014. 86: p. 319-28.
8. LIU X, WANG J, SUN L. Structure of the hyperosmolality-gated calcium-permeable channel OSCA1.2 [J]. Nat Commun, 2018, 9(1): 5060.
References
1. MURTHY S E, DUBIN A E, WHITWAM T, et al. OSCA/TMEM63 are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels [J]. Elife, 2018, 7.
2. FUKUMURA S, HIRAIDE T, YAMAMOTO A, et al. A novel de novo TMEM63A variant in a patient with severe hypomyelination and global developmental delay [J]. Brain and Development, 2022, 44(2): 178-183.
3. VETRO A, BALESTRINI S, PELOROSSO C, et al. Stretch-activated ion channel TMEM63B associates with developmental and epileptic encephalopathies and progressive neurodegeneration [J]. medRxiv, 2022.
4. TABARA L C, AL-SALMI F, MAROOFIAN R, et al. TMEM63C mutations cause mitochondrial morphology defects and underlie hereditary spastic paraplegia [J]. Brain, 2022, 145(9): 3095-3107.
5. ZHANG M, SHAN Y, COX C D, et al. A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity [J]. Nat Commun, 2023, 14(1): 3943.
6. ZHENG W, RAWSON S, SHEN Z, et al. TMEM63 proteins function as monomeric high-threshold mechanosensitive ion channels [J]. Neuron, 2023, 111(20): 3195-3210 e3197.
7. Cameron, C.M., R.M. Wightman, and R.M. Carelli, Dynamics of rapid dopamine release in the nucleus accumbens during goal-directed behaviors for cocaine versus natural rewards. Neuropharmacology, 2014. 86: p. 319-28.
8. LIU X, WANG J, SUN L. Structure of the hyperosmolality-gated calcium-permeable channel OSCA1.2 [J]. Nat Commun, 2018, 9(1): 5060.