March 2024:
RESEARCH HIGHLIGHTSHemei Huang
TACAN is an ion channel-like protein that may be involved in sensing mechanical pain. Here, Chen et al. solved the cryo-EM structures of human TACAN in resting sate, suggesting that each protomer contains a putative ion conduction pore, and combined electrophysiological experiments and molecular dynamics simulations to determine the ion conduction pathway and possible mechanical gating mechanism of TACAN protein. The results showed the molecular assembly of hTACAN and suggest that wild-type hTACAN is in a closed state.
Pain is a biological warning signal that is detected by nociceptors that are activated by multiple environmental factors. It is sensed by the opening of several types of mechanosensitive ion channels.For example, the Piezo mechanosensitive ion, which won the Nobel Prize in 2021, not only transmits tactile sensation but is also related to mechanical force induced pain1. In March 2020, Canadian scientists discovered a new type of ion channel called TACAN, which can sense acute mechanical pain in daily life, such as pain caused by a hammer hitting a finger2. In May 2021, American scientists further utilized a rat model to discover that TACAN plays a role in mechanical pain caused by inflammation but does not play a role in chemotherapy induced mechanical pain3.
However, in August 2021, three articles were published in the same issue of Elife, they both analyzed the Cryo-EM structure of TACAN and unanimously agreed that TACAN may be a coenzyme A binding protein rather than a mechanosensitive ion channel4,5,6. In August 2021, Yan Zhen's research group at West Lake University analyzed the Cryo-EM structures of different isomers of TACAN and published an article in Cell Discovery, suggesting that the biological function of TACAN may be an enzyme in lipid metabolism7. The articles based on these three-dimensional structures challenge the biological function of TACAN as an ion channel, but unfortunately, they have not provided strong evidence to determine the true biological function of TACAN.
Analyzing the Cryo-EM structure of TACAN, the team found that there is a significant similarity between the assembly of TACAN dimer and another mechanosensitive ion channel, OSCA (Plant Osmotic Pressure Sensitive Mechanosensitive Ion Channel) dimer. Molecular dynamics simulation of TACAN revealed that in a resting state, water molecules cannot form continuous pathways on both sides of the protein, indicating that TACAN is in a closed state at this time; When a tension of 35 mN/m is applied to the phospholipid bilayer in a molecular dynamics simulation system, water molecules can pass through the middle pore of the TACAN monomer and form continuous pathways on both sides of the phospholipid bilayer.
Among them, the three amino acid residues M207, F223, and N165 form the limiting regions of the pores. Using electrophysiological techniques to measure current, it was found that the amplitude of current change in wild-type TACAN was not significant in response to membrane tension; However, the current of the M207 mutant of the gated amino acid increased nearly six times in response to membrane tension.
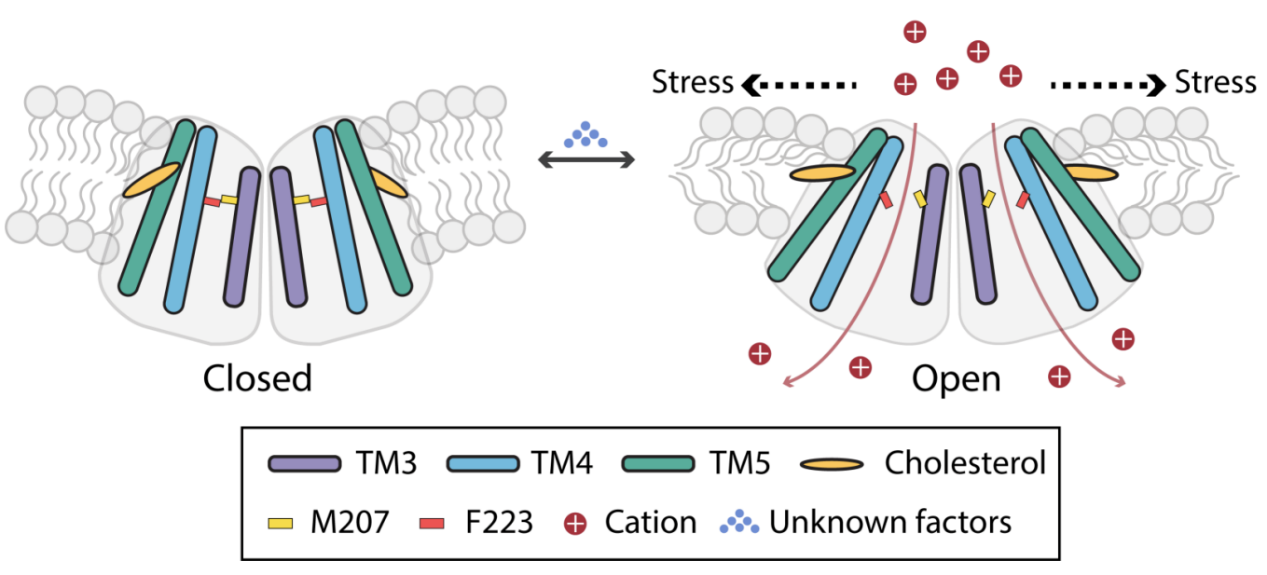
Figure 1 Putative opening mechanism of hTACAN under mechanical stress
Therefore, the team believes that the TACAN protein is in a closed state in a resting state (similar to the results published by other research groups) and may require other unknown cofactors to help the channel transition to another state (such as a pre activated state), which can only be further converted to an open state after sensing mechanical force. The research results currently obtained by the team support TACAN as a novel mechanosensitive ion channel and this dual locking mechanism is consistent with the biological function of TACAN as an acute mechanical pain.
1. Murthy, S. E., Dubin, A. E., and Patapoutian, A. (2017) Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18, 771-783
2. Beaulieu-Laroche, L., Christin, M., Donoghue, A., et al. (2020) TACAN Is an Ion Channel Involved in Sensing Mechanical Pain. Cell 180, 956-967
3. Bonet, I. J. M., Araldi, D., Bogen, O., and Levine, J. D. (2021) Involvement of TACAN, a Mechanotransducing Ion Channel, in Inflammatory But Not Neuropathic Hyperalgesia in the Rat. J Pain 22, 498-508
4. Niu, Y., Tao, X., Vaisey, G., et al. (2021) Analysis of the mechanosensor channel functionality of TACAN. eLife 10, e71188
5. Xue, J., Han, Y., Baniasadi, H., et al. (2021) TMEM120A is a coenzyme A-binding membrane protein with structural similarities to ELOVL fatty acid elongase. eLife 10, e71220
6. Rong, Y., Jiang, J., Gao, Y., et al. (2021) TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells. eLife 10, e71474
7. Ke, M., Yu, Y., Zhao, C., et al. (2021) Cryo-EM structures of human TMEM120A and TMEM120B. Cell Discov 7, 77
References
1. Murthy, S. E., Dubin, A. E., and Patapoutian, A. (2017) Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18, 771-783
2. Beaulieu-Laroche, L., Christin, M., Donoghue, A., et al. (2020) TACAN Is an Ion Channel Involved in Sensing Mechanical Pain. Cell 180, 956-967
3. Bonet, I. J. M., Araldi, D., Bogen, O., and Levine, J. D. (2021) Involvement of TACAN, a Mechanotransducing Ion Channel, in Inflammatory But Not Neuropathic Hyperalgesia in the Rat. J Pain 22, 498-508
4. Niu, Y., Tao, X., Vaisey, G., et al. (2021) Analysis of the mechanosensor channel functionality of TACAN. eLife 10, e71188
5. Xue, J., Han, Y., Baniasadi, H., et al. (2021) TMEM120A is a coenzyme A-binding membrane protein with structural similarities to ELOVL fatty acid elongase. eLife 10, e71220
6. Rong, Y., Jiang, J., Gao, Y., et al. (2021) TMEM120A contains a specific coenzyme A-binding site and might not mediate poking- or stretch-induced channel activities in cells. eLife 10, e71474
7. Ke, M., Yu, Y., Zhao, C., et al. (2021) Cryo-EM structures of human TMEM120A and TMEM120B. Cell Discov 7, 77