April 2024:
RESEARCH HIGHLIGHTSYi Luo
Institute of Molecular Physiology, Shenzhen Bay Laboratory
Abstract:
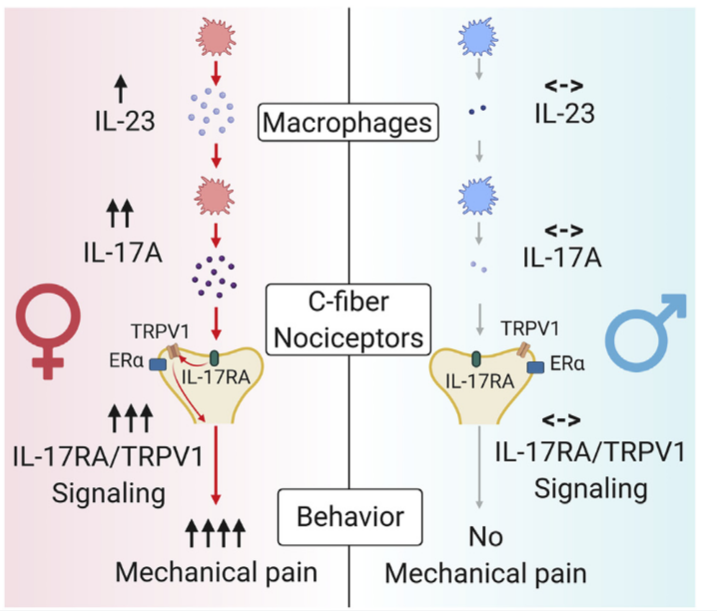
Although sex dimorphism is increasingly recognized as an important factor in pain, female-specific pain signaling is not well studied. The author reports that administration of IL-23 produces mechanical pain (mechanical allodynia) in female but not male mice, and chemotherapy-induced mechanical pain is selectively impaired in female mice lacking Il23 or Il23r. IL-23-induced pain is promoted by estrogen but suppressed by androgen, suggesting an involvement of sex hormones. IL-23 requires C-fiber nociceptors and TRPV1 to produce pain but does not directly activate nociceptor neurons. Notably, IL-23 requires IL-17A release from macrophages to evoke mechanical pain in females. Low-dose IL-17A directly activates nociceptors and induces mechanical pain only in females. Finally, deletion of estrogen receptor subunit a (ERa) in TRPV1+ nociceptors abolishes IL-23- and IL-17-induced pain in females. These findings demonstrate that the IL-23/IL-17A/TRPV1 axis regulates female-specific mechanical pain via neuro-immune interactions.
Text:
Sex dimorphism in chronic pain is a well-recognized clinical phenomenon, as emerging studies suggest sex differences in neuro-immune modulation of pain 1 and females are more likely to suffer from chronic pain conditions 2. Macrophages are increasingly appreciated to play important roles in the pathogenesis and resolution of pain 3 and emerging evidence suggests that male and female macrophages may use distinct pathways to modulate pain 4. The mechanism by which macrophages contribute to pain pathogenesis in females remains unknown.
The author first measured the behavioral effects of intraplantar (i.pl.) injections of IL-23 at various doses in naive male and female mice and following Von Frey testing showed that i.pl. IL-23 produced mechanical allodynia in a dose-dependent manner selectively in females, but not in males. IL-23R is the only known receptor of IL-23 5. Notably, IL-23evoked mechanical allodynia was abolished in Il23r knock out female mice, suggesting a specific effect of IL-23 that is mediated by IL-23R. Furthermore, IL-23-induced mechanical pain in female mice was released by i.pl. injection of the IL23R antagonist P2305.
To examine whether the sexual dimorphism of IL-23-induced pain is specific to behavioral paradigm or generic, the authors further adopted a two-chamber conditioned place aversion (CPA) assay, in which the IL-23 was intrathecally (i.t.) injected into the spinal cord. The results suggest that IL-23 signaling drives mechanical pain through IL-23R selectively in females, but not in males.
Besides the IL-23 injection-induced neuropathic pain, the authors wanted to interrogate whether IL-23 is also involved in inflammatory pain. Therefore, they used a mouse model of chemotherapy-induced peripheral neuropathy (CIPN), by intraperitoneal (i.p.) injecting chemotherapeutic agent paclitaxel (PTX) in both sexes. This CIPN model allowed us to collect DRGs at all the spine levels for various analyses 6. ELISA data revealed a significant increase of IL-23 in serum and DRGs of female CIPN mice compared with naive mice. By using other reagents and P2305, authors got a conclusion that the IL-23/IL-23R axis is required for generating female-specific mechanical allodynia (neuropathic pain) and spontaneous pain (inflammatory pain).
To further understand the mechanisms underlying sex dimorphism of IL-23 signaling in pain, the author used multiple surgical and pharmacological approaches to investigate the impact of estrogen and androgen signaling in this process. Ovariectomy (OVX) and orchiectomy (ORX) could cut off the supply of endogenous hormones. Von Frey test suggests that sex hormones differentially regulate IL-23-mediated mechanical pain by injection of different kinds of agonist and antagonist of sex hormone receptors in males and females. Together, these findings suggest crucial roles of (1) estrogen in promoting IL-23-mediated mechanical pain via ERα and (2) androgen (testosterone) in suppressing this pain.
To assess the cellular mechanisms underlying IL-23-induced pain, the author found that intravenous injection of a macrophages/monocytes toxin to melt the macrophages/monocytes in mice could completely prevented IL-23 evoked mechanical pain. Given a critical role of T cells in mediating IL-23 signaling in disease conditions such as psoriasis 7, the authors conducted similar experiments in nude and Rag knock out mice which were born without T cells. They found that i.pl. IL-23 was fully capable of inducing mechanical allodynia in both lines of immune-deficient mice. These results suggest that macrophages/monocytes, but not T cells, are both required and sufficient to mediate IL-23-produced mechanical pain in females.
In the spinal cord, C-fiber and A-fiber sensory neurons mediate mechanical pain via distinct mechanisms 8. Ablation of TRPV1+ C-fibers by resiniferatoxin (RTX) completely abolished mechanical pain induced by IL-23. The author also blocked C-fibers or A-fibers (Aβ fibers) using QX-314, which is a membrane-impermeable permanently charged sodium channel blocker, together with the C-fiber activator capsaicin and the Ab-fiber activator flagellin. i.pl. pretreatment with QX-314 plus capsaicin, but not QX314 plus flagellin prevented IL-23-evoked mechanical pain in female mice compared with control mice treated with QX314 alone, suggesting the involvement of C-fibers but not Ab fibers in this process. Whole-cell patch clamp recording revealed that IL-23 bath application (100 ng/ mL) did not alter the number of action potential (AP) discharges in response to suprathreshold current injection. Taken together, the evidence indicates that DRG sensory neurons are not directly activated by IL-23.
IL-17A expression is a downstream effector of IL-23 signaling in T cells and macrophages 4 and previous studies have shown pro-nociceptive effects of i.pl. IL-17A in rodents 9. The author observed that PTX increased IL-17A levels in both male and female macrophages. Importantly, IL-23 incubation was sufficient to induce IL-17A secretion in female but not male peritoneal macrophage cultures. Then the author found that IL-17A- evoked mechanical pain in female mice in a dose and time dependent manner. In contrast, only IL-17A at 100 ng produced transient mechanical pain (1 h) in males. Next, the author investigated the contribution of IL-17A to mechanical pain after CIPN in both sexes. PTX enhanced serum IL-17A in both sexes, with slightly higher levels in females.
Collectively, these data suggest that (1) IL-17A production and release in macrophages requires IL-23/IL-23R axis in females, and (2) IL17A is the downstream effector of macrophage IL-23 signaling in female mechanical pain.
The author performed Ca2+ imaging to examine nociceptor activation by IL-17A in DRG sensory neurons dissociated from male and female Advillin-Cre/GCamp6f mice, finding that IL-17A activates mouse sensory neurons in a sex-dependent manner. Electrophysiological results in human and monkey DRG cells confirmed this conclusion
TRPV1 and TRPA1 are critical Ca2+ channels for generating pain 10. Then the author asked whether these channels are required for IL-23-induced nociception using genetic and pharmacological approaches. Notably, Trpv1 deletion abolished mechanical pain induced by i.pl. and i.t. injection of 100 ng IL-23 in females, and i.pl. P2305 failed to reduce PTX-induced mechanical pain in Trpv knock out mice in both sexes. In contrast, i.pl. or i.t. injection of IL-23 effectively evoked mechanical pain in Trpa1knock out female mice, and furthermore, P2305 reduced PTX-evoked mechanical pain only in Trpa1knock out female mice. These findings suggest that TRPV1 but not TRPA1 is indispensable for IL-23induced mechanical pain in females.
Then by crossing Erα-flox mice with Trpv1-Cre mice, the author determined the role of ERα in TRPV1+ nociceptors for mechanical pain.
The authors propose a working model underlying the female-specific modulation of mechanical pain by IL-23/IL-17A/TRPV1 axis. And they noted that both macrophage and nociceptor signaling contribute to the sex dimorphism.
1. Mogil, J.S. (2020). Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 21, 353–365.
2. Fillingim, R.B., King, C.D., Ribeiro-Dasilva, M.C., Rahim-Williams, B., and Riley, J.L., 3rd (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain 10, 447–485..
3. Bang, S., Xie, Y.K., Zhang, Z.J., Wang, Z., Xu, Z.Z., and Ji, R.R. (2018). GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Invest. 128, 3568–3582.
4. Luo, X., Huh, Y., Bang, S., He, Q., Zhang, L., Matsuda, M., and Ji, R.R. (2019c). Macrophage Toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J. Neurosci. 39, 6848–6864.
5. Gaffen, S.L., Jain, R., Garg, A.V., and Cua, D.J. (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600.
6. Liu, X.J., Zhang, Y., Liu, T., Xu, Z.Z., Park, C.K., Berta, T., Jiang, D., and Ji, R.R. (2014). Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 24, 1374–1377.
7. Riol-Blanco, L., Ordovas-Montanes, J., Perro, M., Naval, E., Thiriot, A., Alvarez, D., Paust, S., Wood, J.N., and von Andrian, U.H. (2014).
8. Hill, R.Z., and Bautista, D.M. (2020). Getting in touch with mechanical pain mechanisms. Trends Neurosci. 43, 311–325.
9. Kim, C.F., and Moalem-Taylor, G. (2011). Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 12, 370–383
10. Caterina, M.J., and Julius, D. (2001). The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517.
References
1. Mogil, J.S. (2020). Qualitative sex differences in pain processing: emerging evidence of a biased literature. Nat. Rev. Neurosci. 21, 353–365.
2. Fillingim, R.B., King, C.D., Ribeiro-Dasilva, M.C., Rahim-Williams, B., and Riley, J.L., 3rd (2009). Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain 10, 447–485..
3. Bang, S., Xie, Y.K., Zhang, Z.J., Wang, Z., Xu, Z.Z., and Ji, R.R. (2018). GPR37 regulates macrophage phagocytosis and resolution of inflammatory pain. J. Clin. Invest. 128, 3568–3582.
4. Luo, X., Huh, Y., Bang, S., He, Q., Zhang, L., Matsuda, M., and Ji, R.R. (2019c). Macrophage Toll-like receptor 9 contributes to chemotherapy-induced neuropathic pain in male mice. J. Neurosci. 39, 6848–6864.
5. Gaffen, S.L., Jain, R., Garg, A.V., and Cua, D.J. (2014). The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 14, 585–600.
6. Liu, X.J., Zhang, Y., Liu, T., Xu, Z.Z., Park, C.K., Berta, T., Jiang, D., and Ji, R.R. (2014). Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res. 24, 1374–1377.
7. Riol-Blanco, L., Ordovas-Montanes, J., Perro, M., Naval, E., Thiriot, A., Alvarez, D., Paust, S., Wood, J.N., and von Andrian, U.H. (2014).
8. Hill, R.Z., and Bautista, D.M. (2020). Getting in touch with mechanical pain mechanisms. Trends Neurosci. 43, 311–325.
9. Kim, C.F., and Moalem-Taylor, G. (2011). Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. J. Pain 12, 370–383
10. Caterina, M.J., and Julius, D. (2001). The vanilloid receptor: a molecular gateway to the pain pathway. Annu. Rev. Neurosci. 24, 487–517.