July 2024:
RESEARCH HIGHLIGHTSJianying Dong
Institute of Molecular Physiology, Shenzhen Bay Laboratory
Abstract:
Mechanosensitive (MS) ion channels are ubiquitous and they sense forces from the surrounding bilayer. Here, the authors determined the structures of plant and mammalian OSCA/TMEM63 proteins, identifying essential elements for mechanotransduction and elucidating the mechanosensitive function of bounding lipids. Briefly, the central cavity created by the dimer interface couples each subunit. Mechanosensitivity of the dimeric OSCA/TMEM63 channel is modulated through a modulating lipids, and the cytosolic side of the pore is gated by a plug lipid that prevents the ion permeation. Our results suggest that the gating mechanism of OSCA/TMEM63 channels may combine structural features of the ‘lipid-gated’ mechanism of MscS and TRAAK channels and the calcium-induced gating mechanism of theTMEM16 family. These results may provide insights into the structural rearrangements of TMEM16/TMC superfamilies.
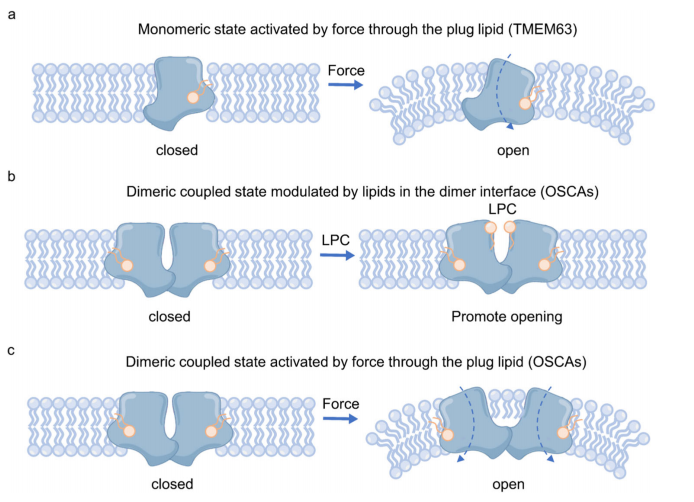
The putative mechanical-coupling mechanism of OSCA/TMEM63 channels
Text:
Mechanosensitive ion channels, which are molecular entities directly sensing changes in external forces, are widely distributed in living organisms. OSCA was originally discovered through screening for osmotic stress response defects in plants and subsequently shown to respond to osmotic pressure when expressed heterologously in Xenopus oocytes 1,2, featuring in its higher threshold of pressure compared to that of Piezo. OSCA/TMEM63 was resolved at atomic resolution using cryo-EM 3. It forms a dimeric transporter-like configuration, which is similar to the calcium-activated chloride channel TMEM16 and auditory-related TMC family members4,5. However, due to technical challenges for preparing cryo-EM samples with high pressure, structural and functional understandings of its mechanism remains unsatisfactory.
The authors purified the human OSCA/TMEM63A protein and determined its high-resolution cryo-EM structure, revealing that it exists as a monomer rather than the previously reported dimer. Furthermore, the cryo-EM structure clearly demonstrated that a phospholipid molecule obstructs ion flow. Interestingly, the phospholipid occupies a position analogous to where the calcium ion activates chloride channels in TMEM16A. Moreover, the lipid-blocking mechanism also mirrors those observed in bacterial mechanosensitive ion channel MscS and the mammalian mechanosensitive potassium channel TRAAK. Thus, OSCA/TMEM63 integrates gating characteristics of TMEM16A, MscS, and TRAAK. Further experiments are warranted to validate the preservation of these gating features in the dimeric form of human OSCA/TMEM63.
The authors re-purified the Arabidopsis thaliana OSCA1.1 (AtOSCA1.1) protein, incorporated it into nanodiscs, and resolved the cryo-EM structures with a higher-resolution of 2.5 Å. Compared to the previous structure in detergent environments, the authors noted slight discrepancies as the central interaction region of the dimer in the lipid membrane exhibited an open conformation. They also found that this expansion might be facilitated by endogenous hemolytic phospholipids, corroborating earlier evidence suggesting these phospholipids can enhance channel activation. Furthermore, in this high-resolution cryo-EM structure, the authors observed phospholipid molecules in the single-pore region acting as potential gating elements, reminiscent of the lipid-binding site observed in human OSCA/TMEM63A. This indicates that the lipid gating properties are preserved in the dimeric form of the OSCA/TMEM63 channel.
The authors then revealed the high-resolution structure of AtOSCA3.1, which displays an enhanced dimeric response to higher pressures. They also observed phospholipid molecules binding in the single-pore region of AtOSCA3.1. Notably, AtOSCA3.1 exhibited distinct open and closed forms at its dimeric interface. The closed form is facilitated by interactions between the fifth and sixth transmembrane segments of each subunit. The authors hypothesize that the transition from the closed to open form of OSCA3.1 likely involves overcoming an energy barrier imposed by these interactions, suggesting that higher pressures are necessary to activate this transition.
In summary, relying on three sets of high-resolution cryo-EM structures, the authors have proposed a force transmission gating mechanism through dimeric interactions, expanding upon the mechanosensitive gating mechanism involving lipid-blocked pores. This introduces a novel perspective on understanding the mechanosensitivity and transmission of mechanical force in ion channels.
Link to the research article:
https://pubmed.ncbi.nlm.nih.gov/37402734/
1. Yuan F., Yang H,. Xue Y., Kong D., Ye R., Li C., Zhang J., Theprungsirikul L., Shrift T., Krichilsky B., Johnson D.M., Swift G.B., He Y., Siedow J.N., Pei Z.M. (2014). OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514, 367-371 .
2. Hou C., Tian W., Kleist T., He K., Garcia V., Bai F., Hao Y., Luan S., Li L. (2014). DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 24, 632-635.
3. Zhang M., Wang D., Kang Y., Wu J.X., Yao F., Pan C., Yan Z., Song C., Chen L. (2018). Structure of the mechanosensitive OSCA channels. Nat. Struct. Mol. Biol. 25, 850-858.
4. Dang S., Feng S., Tien J., Peters C.J., Bulkley D., Lolicato M., Zhao J., Zuberbühler K., Ye W., Qi L., Chen T., Craik C.S., Jan Y.N., Minor D.L. Jr, Cheng Y., Jan L.Y. (2017). Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426-429.
5. Jeong H., Clark S., Goehring A., Dehghani-Ghahnaviyeh S., Rasouli A., Tajkhorshid E., Gouaux E. (2022). Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature 610, 796-803.
References
1. Yuan F., Yang H,. Xue Y., Kong D., Ye R., Li C., Zhang J., Theprungsirikul L., Shrift T., Krichilsky B., Johnson D.M., Swift G.B., He Y., Siedow J.N., Pei Z.M. (2014). OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514, 367-371 .
2. Hou C., Tian W., Kleist T., He K., Garcia V., Bai F., Hao Y., Luan S., Li L. (2014). DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 24, 632-635.
3. Zhang M., Wang D., Kang Y., Wu J.X., Yao F., Pan C., Yan Z., Song C., Chen L. (2018). Structure of the mechanosensitive OSCA channels. Nat. Struct. Mol. Biol. 25, 850-858.
4. Dang S., Feng S., Tien J., Peters C.J., Bulkley D., Lolicato M., Zhao J., Zuberbühler K., Ye W., Qi L., Chen T., Craik C.S., Jan Y.N., Minor D.L. Jr, Cheng Y., Jan L.Y. (2017). Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426-429.
5. Jeong H., Clark S., Goehring A., Dehghani-Ghahnaviyeh S., Rasouli A., Tajkhorshid E., Gouaux E. (2022). Structures of the TMC-1 complex illuminate mechanosensory transduction. Nature 610, 796-803.