May 2024:
RESEARCH HIGHLIGHTSSiqi DENG
Molecular Physiology, Shenzhen Bay Laboratory
Abstract: The OSCA/TMEM63 channels are the largest family of mechanosensitive channels known to play crucial roles in mechanotransduction in both plants and mammals. This study investigated the molecular basis of OSCA/TMEM63 channel mechanosensitivity by obtaining cryogenic electron microscopy structures of these channels in different environments 1. The authors mimicked increased membrane tension in nanodiscs and observed a dilated pore with membrane access in one of the OSCA1.2 subunits. In liposomes, they captured the fully open structure of OSCA1.2 in the inside-in orientation where the pore exhibited a large lateral opening to the membrane, supporting the model of ‘proteo-lipidic pore’. In the less tension-sensitive homologue OSCA3.1, the authors identified an interlocking lipid that tightly bound in the central cleft and mutation of the lipid-coordinating residues induced OSCA3.1 activation. Overall, these findings expand our knowledge of channel-mediated mechanotransduction and channel pore formation, with important mechanistic implications for the TMEM16 and TMC protein families.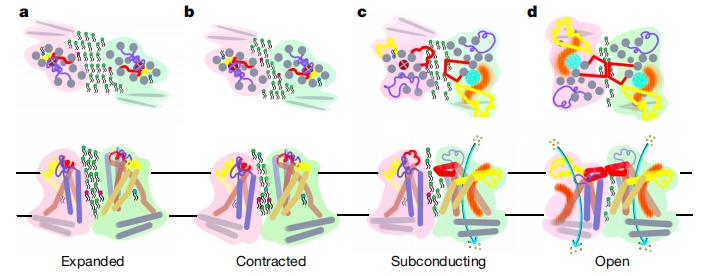
Text:
Mechanical force signals evoke a variety of sensations in different organs, such as the touch is generated from the skin and the sound is generated in the ear. These stimulations are sensed and transmitted mainly through mechanical force sensitive ion channels, converting mechanical force signals into electrochemical signals. The OSCA/TMEM63 family is the largest family of mechanically sensitive ion channels known to date, and plays important physiological functions in both plants and animals. OSCA channels, first identified in the model plant Arabidopsis thaliana, are activated by changes in osmolarity and play important roles in abiotic stress resistance 2,3. In higher organisms, their close homologues TMEM63 channels have been found to participate in many processes including hearing 4, humidity 5 and food texture sensation 6. Despite that the structures of OSCA/TMEM63 channels in a close state have been elucidated3,7,8,9, the molecular mechanism of their mechanosensation remains elusive due to a lack of structural studies of these channels under mechanical force.
To address this question, the authors employed a variety of methods to manipulate the mechanical environment and utilized cryo-EM to visualize force-induced structural changes of OSCA channels. OSCA channels form homodimers with two subunits that interact exclusively through the cytoplasmic domain. Each subunit has a putative self-contained conduction pathway formed by transmembrane (TM) helices, resulting in a two-pore configuration. Like other inherently mechanosensitive channels, OSCA channels respond to increases in membrane tension. To elucidate the structure of active OSCA channels under tension within nanodiscs, the authors employed two distinct strategies: firstly, by utilizing cyclodextrins to extract lipids from protein-embedded nanodiscs, and secondly, by augmenting the membrane scaffold protein (MSP) to lipid ratio. With increased membrane tension on proteins in structural studies, they identified a ‘smeared’ half in the map of homodimer, showing a profound rearrangement of the TM helices. These movements and dynamics of the helices provide the potential for ion permeation, which was further experimentally validated by proteoliposome recording.
Building upon prior research that leveraged local curvature to induce membrane stress and activate mechanosensitive channels 10, the author successfully customized small diameter proteoliposomes (d ≤ 40 nm) and unveiled the activating structure of OSCA. Notably, this structure also represents the smallest membrane protein resolved in liposomes with high resolution. OSCA1.2 has both inside-out and inside-in orientations in liposomes, both of which contributes to half of the particles. While the inside-out structure resembles a typical nanodisc-embedded closed state of OSCA1.2, there is profound conformational changes in the inside-in structure with both of pores in the homodimer clearly dilated. The residues on the tips of the pore-forming helixes all move apart and the extracellular domain between TM5–6 bends towards down and lays on the membrane. On the cytoplasmic side, the contraction of H3-H4 may be further transduce a dragging force on TM6, resulting in a wider opening. The pore region showed a wide lateral opening to the membrane, which may be the membrane wall formed by head groups from roughly 6–7 lipids revealed by molecular dynamics simulations. Further studies in the structural differences in OSCA1.1, OSCA1.2 and OSCA3.1 reveal the role of bound lipids in OSCA mechanosensation. OSCA3.1 bound more lipids compared to the other members, and majority of bound lipids locate at the pore exit and within the central cleft. Structural and functional studies showed that mutations on the residues interacting with these lipids contributed to an open state of OSCA3.1. These results suggest that the bound lipids in the cleft are a crucial for OSCA3.1 channel gating, thus they are named the ‘interlocking’ lipid.
In light of the structural and functional studies, these results captured the 3D conformation of the activated state of OSCA protein through a variety of different strategies. They first mimicked increased membrane tension in nanodiscs by increasing the MSP to lipid ratio, and observed a dilated pore with membrane access in one of the OSCA1.2 subunits. They further obtained the high-resolution three-dimensional structure of fully-opened OSCA proteins in liposomes. With the help of these two strategies, the important role of different phospholipids in the mechanical force perception of OSCA was revealed. This study not only expands the understanding of ion channel pore architecture, but also provides important mechanistic insights into the functional cycles of the structurally similar TMEM16 and TMC protein families.
1. Han Y, Zhou Z, Jin R, Dai F, Ge Y, Ju X, Ma X, He S, Yuan L, Wang Y, Yang W, Yue X, Chen Z, Sun Y, Corry B, Cox CD, Zhang Y. Mechanical activation opens a lipid-lined pore in OSCA ion channels. Nature. 2024 Apr 3.
2. Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, Johnson DM, Swift GB, He Y, Siedow JN, Pei ZM. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014 Oct 16;514
3. Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, Mousavi SAR, Ward AB, Patapoutian A. OSCA/TMEM63 are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels. Elife. 2018 Nov 1;7:e41844.
4. Du H, Ye C, Wu D, Zang YY, Zhang L, Chen C, He XY, Yang JJ, Hu P, Xu Z, Wan G, Shi YS. The Cation Channel TMEM63B Is an Osmosensor Required for Hearing. Cell Rep. 2020 May 5;31(5):107596.
5. Li S, Li B, Gao L, Wang J, Yan Z. Humidity response in Drosophila olfactory sensory neurons requires the mechanosensitive channel TMEM63. Nat Commun. 2022 Jul 2;13(1):3814.
6. Yang, G. et al. TMEM63B channel is the osmosensor required for thirst drive of interoceptive neurons. Cell discovery (2024).
7. Zhang M, Wang D, Kang Y, Wu JX, Yao F, Pan C, Yan Z, Song C, Chen L. Structure of the mechanosensitive OSCA channels. Nat Struct Mol Biol. 2018 Sep;25(9):850-858.
8. Zheng W, Rawson S, Shen Z, Tamilselvan E, Smith HE, Halford J, Shen C, Murthy SE, Ulbrich MH, Sotomayor M, Fu TM, Holt JR. TMEM63 proteins function as monomeric high-threshold mechanosensitive ion channels. Neuron. 2023 Oct 18;111(20):3195-3210.e7.
9. Zhang M, Shan Y, Cox CD, Pei D. A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity. Nat Commun. 2023 Jul 4;14(1):3943. doi: 10.1038/ s41467-023-39688-8.
10. Yang G, Jia M, Li G, Zang YY, Chen YY, Wang YY, Zhan SY, Peng SX, Wan G, Li W, Yang JJ, Shi YS. TMEM63B channel is the osmosensor required for thirst drive of interoceptive neurons. Cell Discov. 2024 Jan 3;10(1):1.
References
1. Han Y, Zhou Z, Jin R, Dai F, Ge Y, Ju X, Ma X, He S, Yuan L, Wang Y, Yang W, Yue X, Chen Z, Sun Y, Corry B, Cox CD, Zhang Y. Mechanical activation opens a lipid-lined pore in OSCA ion channels. Nature. 2024 Apr 3.
2. Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C, Zhang J, Theprungsirikul L, Shrift T, Krichilsky B, Johnson DM, Swift GB, He Y, Siedow JN, Pei ZM. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature. 2014 Oct 16;514
3. Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, Mousavi SAR, Ward AB, Patapoutian A. OSCA/TMEM63 are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels. Elife. 2018 Nov 1;7:e41844.
4. Du H, Ye C, Wu D, Zang YY, Zhang L, Chen C, He XY, Yang JJ, Hu P, Xu Z, Wan G, Shi YS. The Cation Channel TMEM63B Is an Osmosensor Required for Hearing. Cell Rep. 2020 May 5;31(5):107596.
5. Li S, Li B, Gao L, Wang J, Yan Z. Humidity response in Drosophila olfactory sensory neurons requires the mechanosensitive channel TMEM63. Nat Commun. 2022 Jul 2;13(1):3814.
6. Yang, G. et al. TMEM63B channel is the osmosensor required for thirst drive of interoceptive neurons. Cell discovery (2024).
7. Zhang M, Wang D, Kang Y, Wu JX, Yao F, Pan C, Yan Z, Song C, Chen L. Structure of the mechanosensitive OSCA channels. Nat Struct Mol Biol. 2018 Sep;25(9):850-858.
8. Zheng W, Rawson S, Shen Z, Tamilselvan E, Smith HE, Halford J, Shen C, Murthy SE, Ulbrich MH, Sotomayor M, Fu TM, Holt JR. TMEM63 proteins function as monomeric high-threshold mechanosensitive ion channels. Neuron. 2023 Oct 18;111(20):3195-3210.e7.
9. Zhang M, Shan Y, Cox CD, Pei D. A mechanical-coupling mechanism in OSCA/TMEM63 channel mechanosensitivity. Nat Commun. 2023 Jul 4;14(1):3943. doi: 10.1038/ s41467-023-39688-8.
10. Yang G, Jia M, Li G, Zang YY, Chen YY, Wang YY, Zhan SY, Peng SX, Wan G, Li W, Yang JJ, Shi YS. TMEM63B channel is the osmosensor required for thirst drive of interoceptive neurons. Cell Discov. 2024 Jan 3;10(1):1.